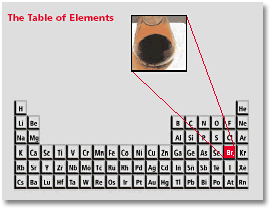
Br Element Name

It is the third lightest halogen and is a fuming red brown liquid at room temperature that evaporates readily to form a similarly coloured gas.
Br element name. Alkali metals alkaline earth metals transition metals other metals metalloids non metals halogens noble gases rare earth elements basic information atomic structure isotopes related links citing this page. Generally the br element should only be used if the line break itself is an intrinsic part of the content. 79 904 amu melting point 7 2 c 265 95. Creating separate paragraphs of text using br is not only bad practice it is problematic for people who navigate with the aid of screen reading technology.
Natural salt deposits and brines are the main sources of bromine and its compounds. Common chemical compounds are also provided for many elements. Including scores of properties element names in many languages most known nuclides of bromine. The br element creates a line break.
Screen readers may announce the presence of the element but not any content contained within br s this can be a confusing and frustrating experience for the person using the screen reader. Bromine chemical element a deep red noxious liquid and a member of the halogen elements or group 17 of the periodic table. He realised this was a new element and reported it to the french academy who confirmed his discovery. Periodic table of elements element bromine br.
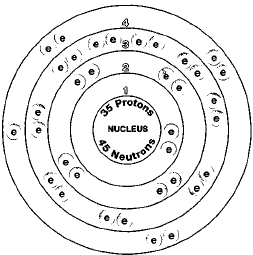
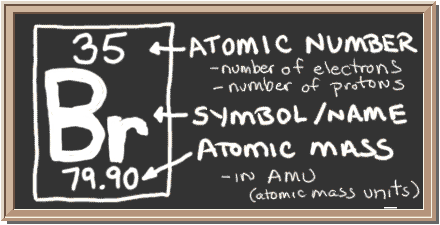
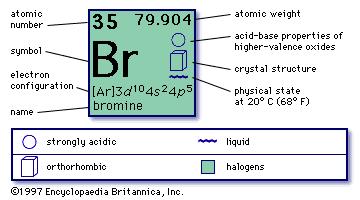
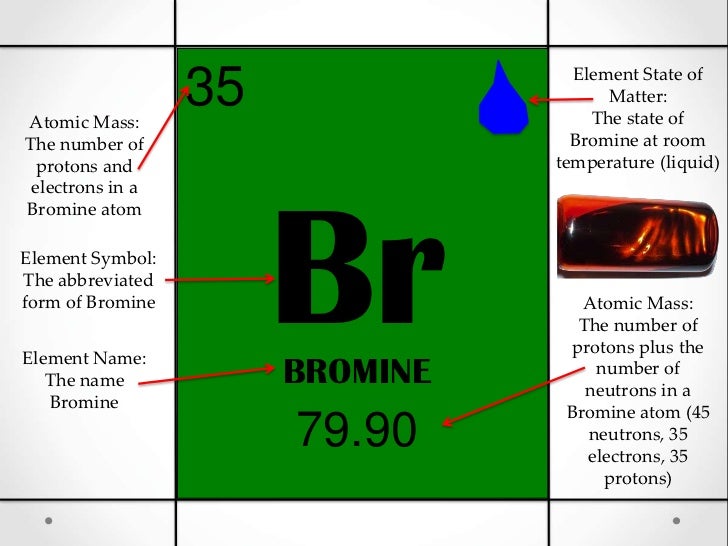
He found that the salt residues left by evaporating brine from montpellier france gave an oily red liquid when treated with acid. Organizing the elements to help further our understanding was first provided by dmitri mendeleev. Jordan israel china and the united states are major producers of bromine. Bromine is a chemical element with the symbol br and atomic number 35.
For quick reference go to the periodic table chart with names listed alphabetical order. The bromine story began with 24 year old student antoine jérôme balard. Its properties are thus intermediate between those of chlorine and iodine isolated independently by two chemists carl jacob löwig in 1825 and antoine jérôme balard in 1826.