Br Periodic Table Charge

You can use this chart to predict whether or not an atom can bond with another atom the charge on an atom is related to its valence electrons or oxidation state an atom of an element is most stable when its outer electron shell is completely filled or half filled.
Br periodic table charge. There are several exceptions to this rule. This can create an overall charge of zero making a compound that is electrically neutral and. Since it is bonded to hydrogen it only has one bond. Bromine is extracted by electrolysis from natural bromine rich brine deposits in the usa israel and china.
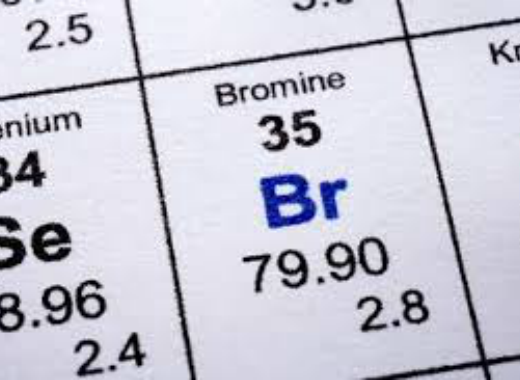
It was the first element to be extracted from seawater but this is now only economically viable at the dead sea israel which is particularly rich in bromide up to 0 5. Since it only has one bond bromine still have 6 electrons surrounding it in the lewis structure. This periodic table with charges is a useful way to keep track of the most common oxidation numbers for each element. Its properties are thus similar to those of fluorine chlorine and iodine and tend to be intermediate between those of the two neighbouring halogens chlorine and iodine bromine has the electron configuration ar 3d 10 4s 2 4p 5 with the seven electrons in the fourth and outermost shell acting as its valence.
Generally metals on the periodic table of the elements have a positive charge a positive ion and the nonmetals have a negative charge a negative ion. So the of valence electrons of bromine is 7. Formal charge is the of valence electrons of bonds of electrons surrounding br in the lewis structure. If you look at the periodic table you will find the metals in groups from one to 16.
With a standard atomic weight of circa 1 008 hydrogen is the lightest element on the periodic table. Each element square contains all 118 of elements with the element number symbol name atomic mass and most common oxidation number. Group one is composed of metals that have a 1 charge while all the metals in groups 2 3 4 5 6 7 8 9 10 11 12 and 16 have a charge 2. Bromine is the third halogen being a nonmetal in group 17 of the periodic table.
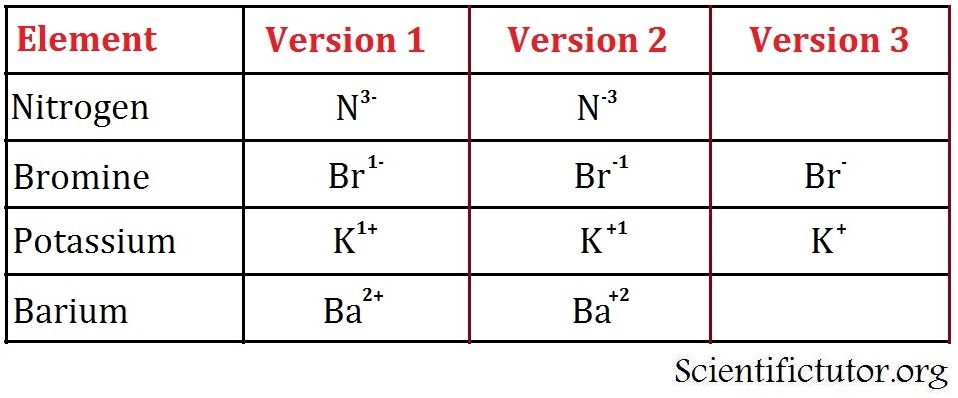
Br 1 2 3 3 2 1 c 4 ion charges predicted from periodic table hydride carbide nitride oxide fluoride phosphide sulfide chloride selenide bromide iodide h ra ca ba sr mg be magnitude of charge given first followed by or 1 and 1 given as and. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure the chemical symbol for hydrogen is h. Kind of confused when you state this. The modern periodic table of elements or just periodic table is a tabular representation of the identified elements as of date 118 along with their respective symbolic name and atomic number.
This is a chart of the most common charges for atoms of the chemical elements. A chemical reaction can take place when a positively charged element meets a negatively charged element.