Br Element Protons

35 number of neutrons.
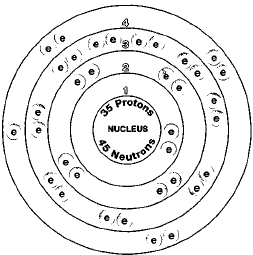
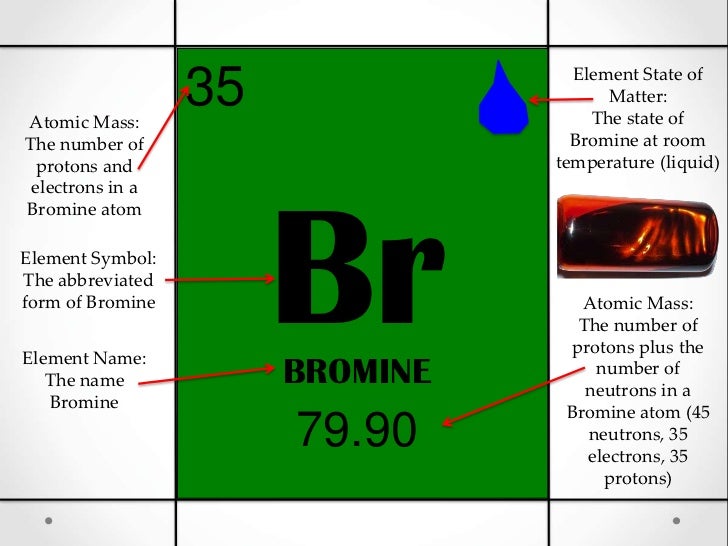
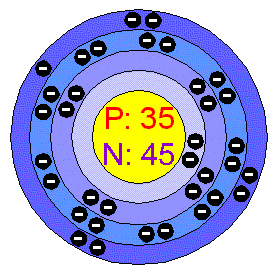
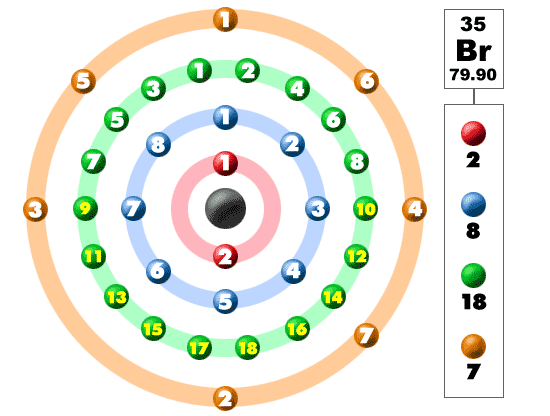
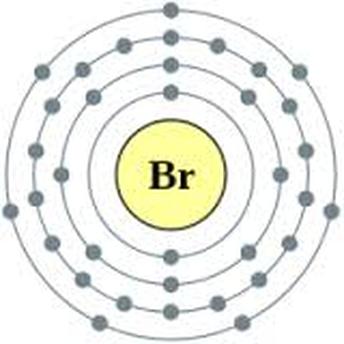
Br element protons. The chemical symbol for bromine is br. Bromine is a chemical element with atomic number 35 which means there are 35 protons and 35 electrons in the atomic structure. Bromine is the third lightest halogen and is a fuming red brown liquid at room temperature that evaporates readily to form a similarly coloured gas. List of isotopes edit nuclide.
From the greek work bromos meaning stench. The number of protons in an element is always the same as the atomic number which is 35 in this case. The chemical symbol for bromine is br. Rare earth elements basic information atomic structure isotopes related links citing this page.
79 904 amu melting point 7 2 c 265 95 k 19 04 f boiling point. Date and place of discovery. It was the first element to be extracted from seawater but this is now only economically viable at the dead sea israel which is particularly rich in bromide up to 0 5. 58 78 c 331 93 k 137 804 f number of protons electrons.
Bromine is a chemical element with atomic number 35 which means there are 35 protons and 35 electrons in the atomic structure. There are 35 protons and 36 electrons in bromide ion the number of neutrons depends on the isotope. In 1825 at the university of heidelberg in germany and simultaneously at the laboratory of medicine and chemistry in montpellier france. In a neutral atom no charge the number of electrons equals the number of protons.
Bromine is a chemical element with atomic number 35 which means there are 35 protons and 35 electrons in the atomic structure. Bromine 35 br has two stable isotopes 79 br and 81 br and 30 known radioisotopes the most stable of which is 77 br with a half life of 57 036 hours. Br 79 and br 81 isotope of bromine will have 44 and 46 neutrons respectively. Bromine is extracted by electrolysis from natural bromine rich brine deposits in the usa israel and china.




.PNG)