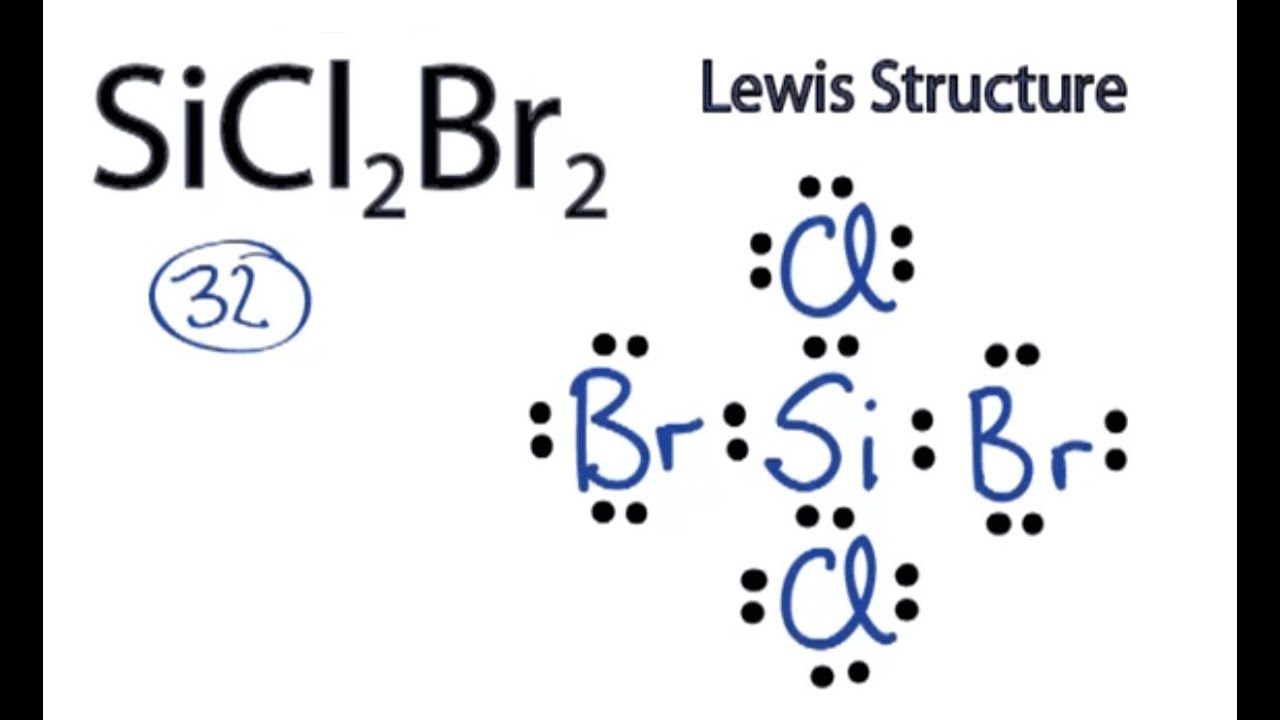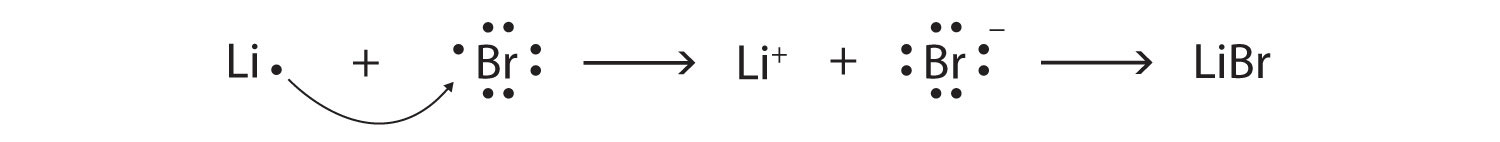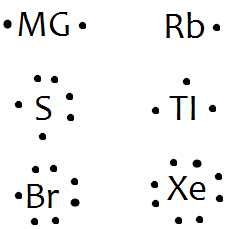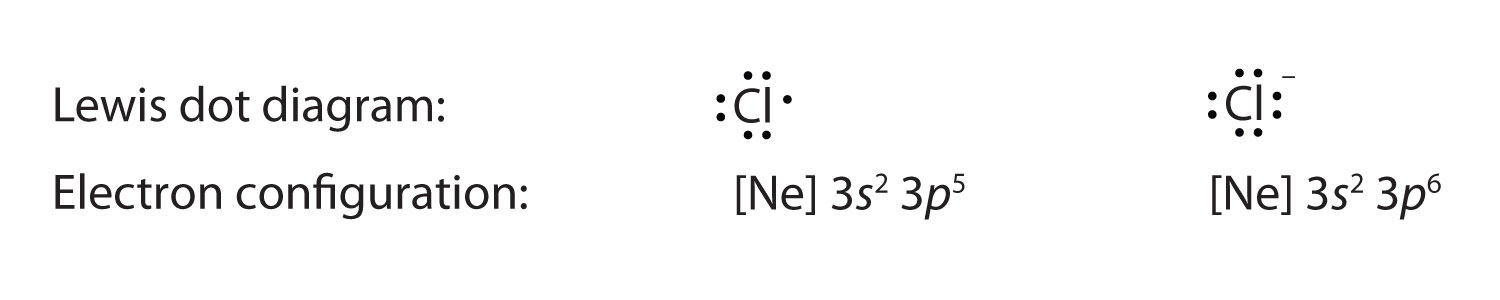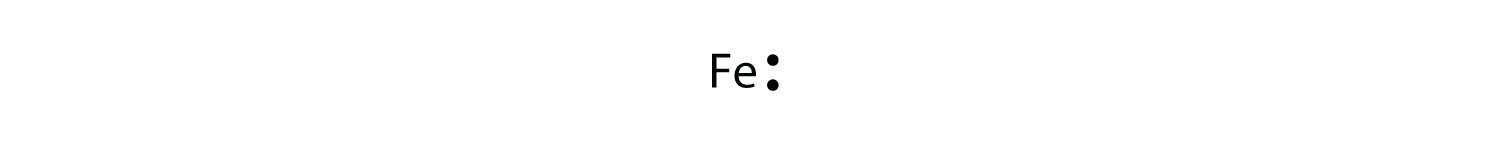Br Element Lewis Structure

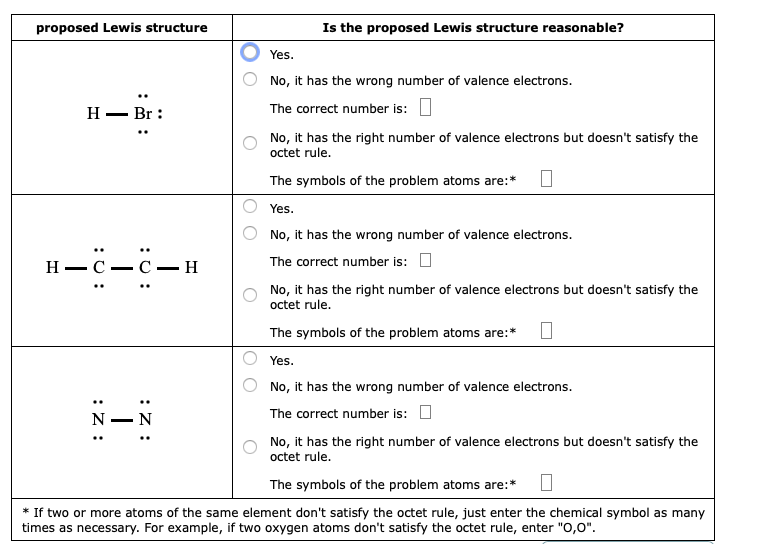
A lewis dot structure is drawn by a series of dots lines and atomic symbols and provides a structure for the way that the atom or molecule is arranged.
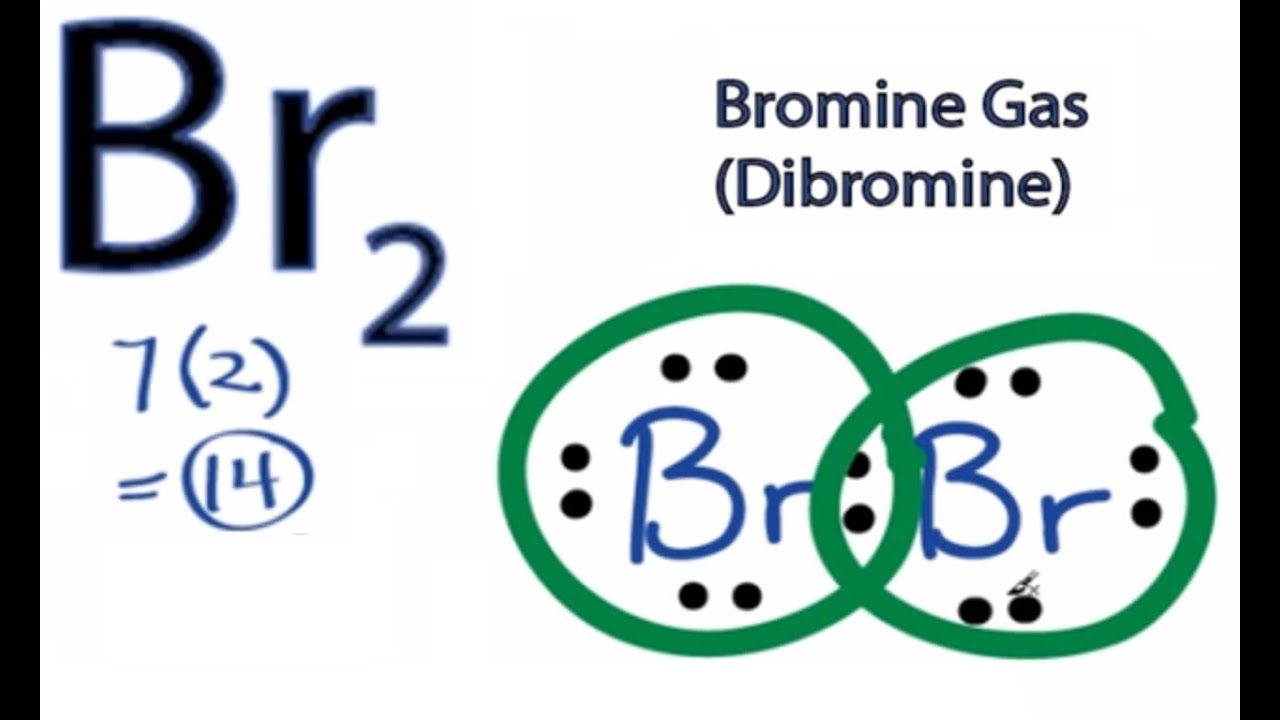
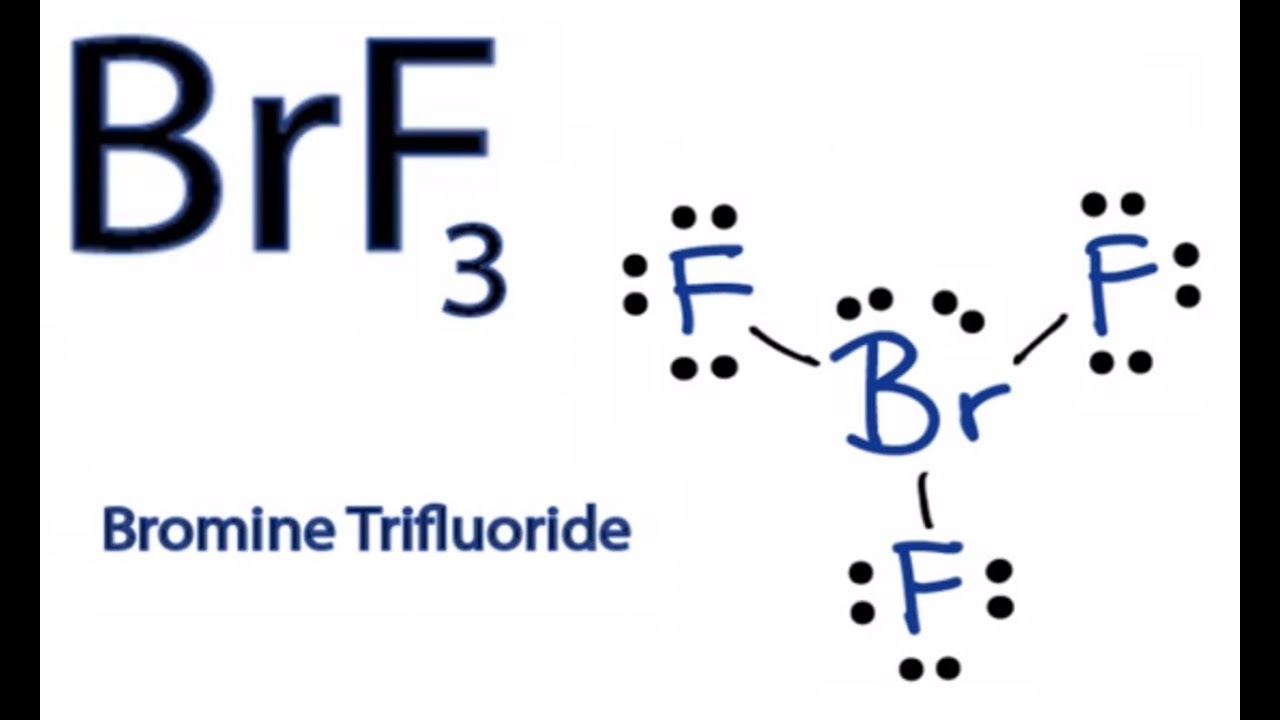
Br element lewis structure. And on the outside we ll put the bromine. Lewis structures are a useful way to summarize certain information about bonding and may be thought of as electron bookkeeping. Using the periodic table to draw lewis dot structures. The other halogen molecules f 2 br 2 i 2 and at 2 form bonds like those in the chlorine molecule.
In lewis dot structures each dot represents an electron. A lewis dot structure can be made for a single atom a covalent compound or a polyatomic ion. Carbon that s the least electronegative that ll go in the center. Similarly lewis diagrams for all elements in other groups such as the alkaline earths or halogens look the same.
Lewis dot structures reflect the electronic structures of the elements including how the electrons are paired. Hence the lewis dot structure is. There are five valence electrons in an atom of nitrogen. Bromine is an active ingredient in four products.
Lewis structures also known as lewis dot structures or electron dot structures are diagrams that represent the valence electrons of atoms within a molecule. Figure pageindex 1 the image above demonstrates that for elements in the same group like the alkali earth metals shown above the lewis dot structure will be the same except of course for the different element name. The dimeric form of aluminium tribromide al 2 br 6 predominates in the solid state in solutions in noncoordinating solvents e g. This is the cbr4 lewis structure.
So 4 plus 28 equals 32 total valence electrons. Hence the lewis dot structure is. Write lewis symbols for the following atoms and ions. These lewis symbols and lewis structures help visualize the valence electrons of atoms and molecules whether they exist as lone pairs or within bonds.
The species aluminium monobromide forms from the reaction of hbr with al metal at high temperature. A pair of dots between. A b c d write the lewis structure for the diatomic molecule p 2 an unstable form of phosphorus found in high temperature phosphorus vapor. One single bond between atoms and three lone pairs of electrons per atom this allows each halogen atom to have a noble gas electron configuration.
The multiple active ingredient products control mold mildew fungi insects and odors in exposed surfaces of bedding mattresses textiles drapes upholstered furniture rugs carpets and storage areas. Bromine in group 7 or 17 so it has 7 and we have 4 bromines. In the lewis structures listed here m and x represent various elements in the third period of the periodic table. The tendency of main group atoms to form enough bonds to obtain eight valence electrons is known as the octet rule.
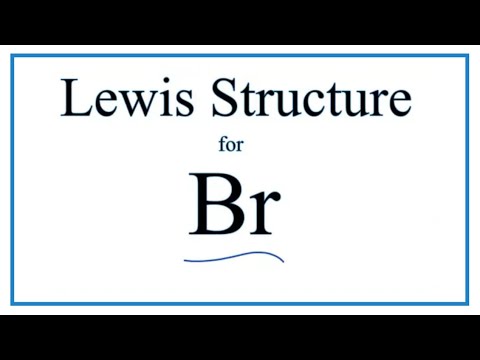
There are seven valence electrons in bromine. There are six valence electrons in an atom of oxygen. Write the formula of each compound using the chemical symbols of each element. Hence the lewis dot structure is.
Cs 2 in the melt and in the gas phase only at high temperatures do these dimers break up into monomers.