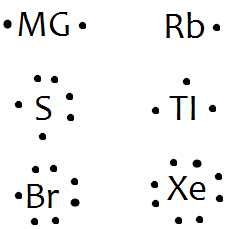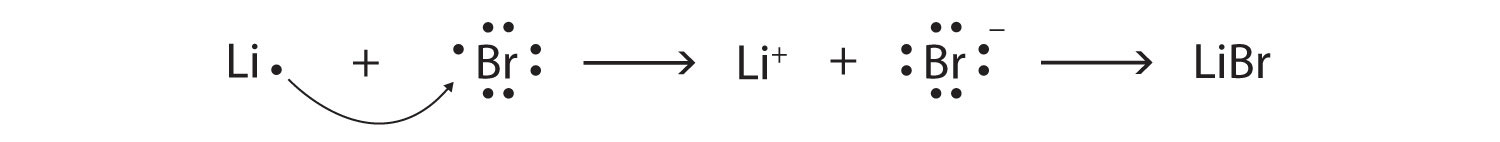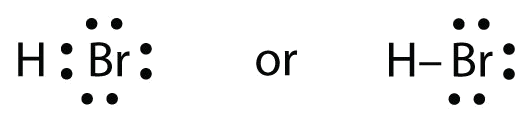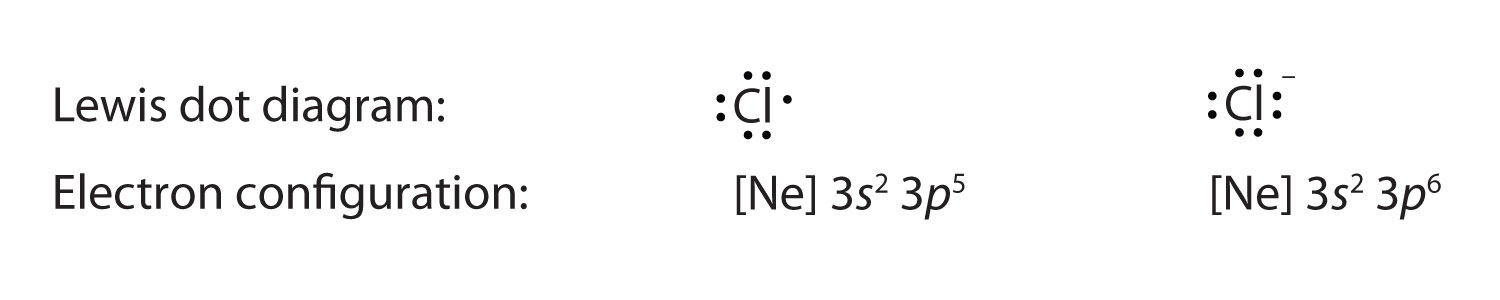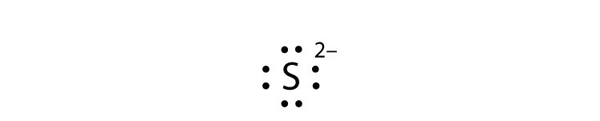Br Element Lewis Dot Structure
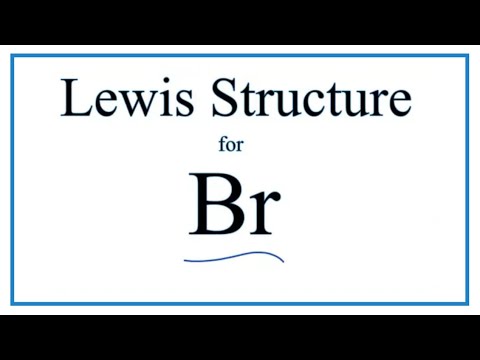
For the br structure use the periodic table to find the total number of valence electro.
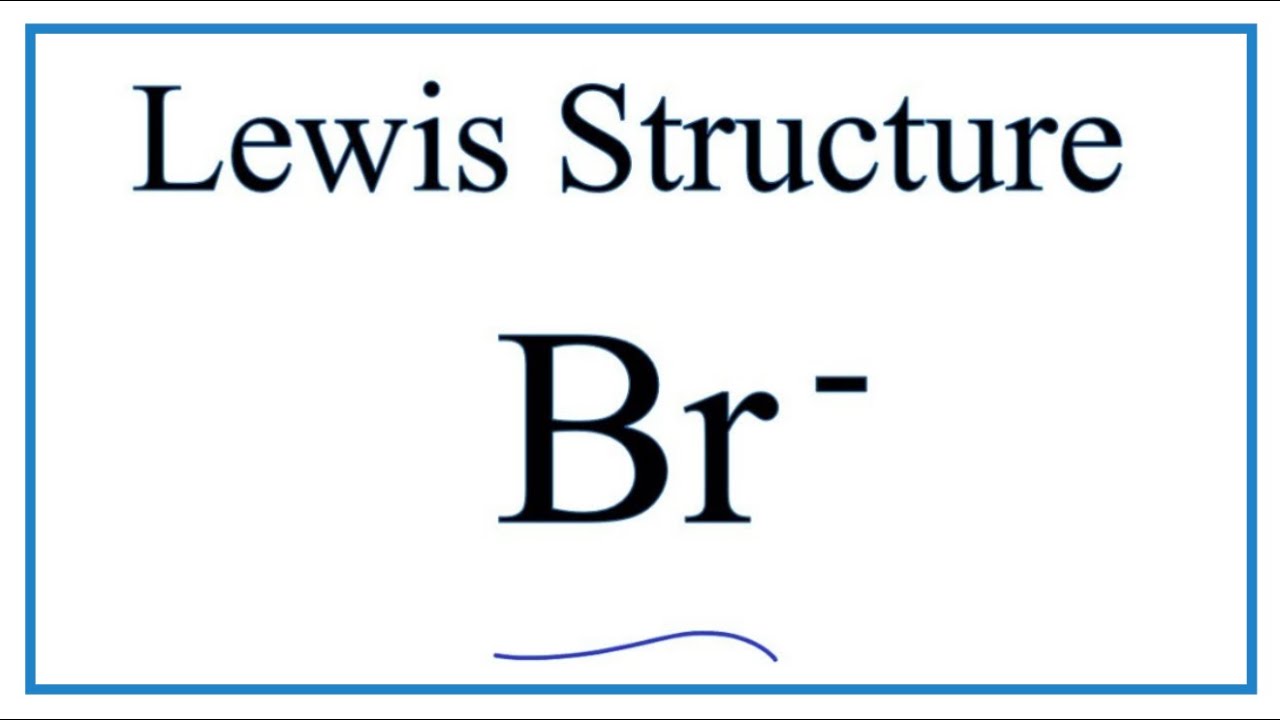
Br element lewis dot structure. The first shell n 1 can have only 2 electrons so that shell is filled in helium the first noble gas. Each bromine br atom contributes 4 valence electrons and the negative sign provides an additional valence electron for a total of 22. A step by step explanation of how to draw the br lewis dot structure for the br structure use the periodic table to find the total number of valence electrons. These lewis symbols and lewis structures help visualize the valence electrons of atoms and molecules whether they exist as lone pairs or within bonds.
A step by step explanation of how to draw the br lewis dot structure. See the following examples for how to draw lewis dot structures for common atoms involved in covalent bonding. Be sure to put brackets and a negative sign around the dot structure to show that br 3. The br 3 lewis structure has a total of 22 valence electrons.
Lewis dot structures reflect the electronic structures of the elements including how the electrons are paired. This type of lewis dot structure is represented by an atomic symbol and a series of dots. A lewis electron dot diagram or electron dot diagram or a lewis diagram or a lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Bromine br has seven valence electrons.
Including scores of properties element names in many languages most known nuclides of bromine. Periodic table of elements element bromine br. Lewis structures also known as lewis dot structures or electron dot structures are diagrams that represent the valence electrons of atoms within a molecule. In lewis dot structures each dot represents an electron.
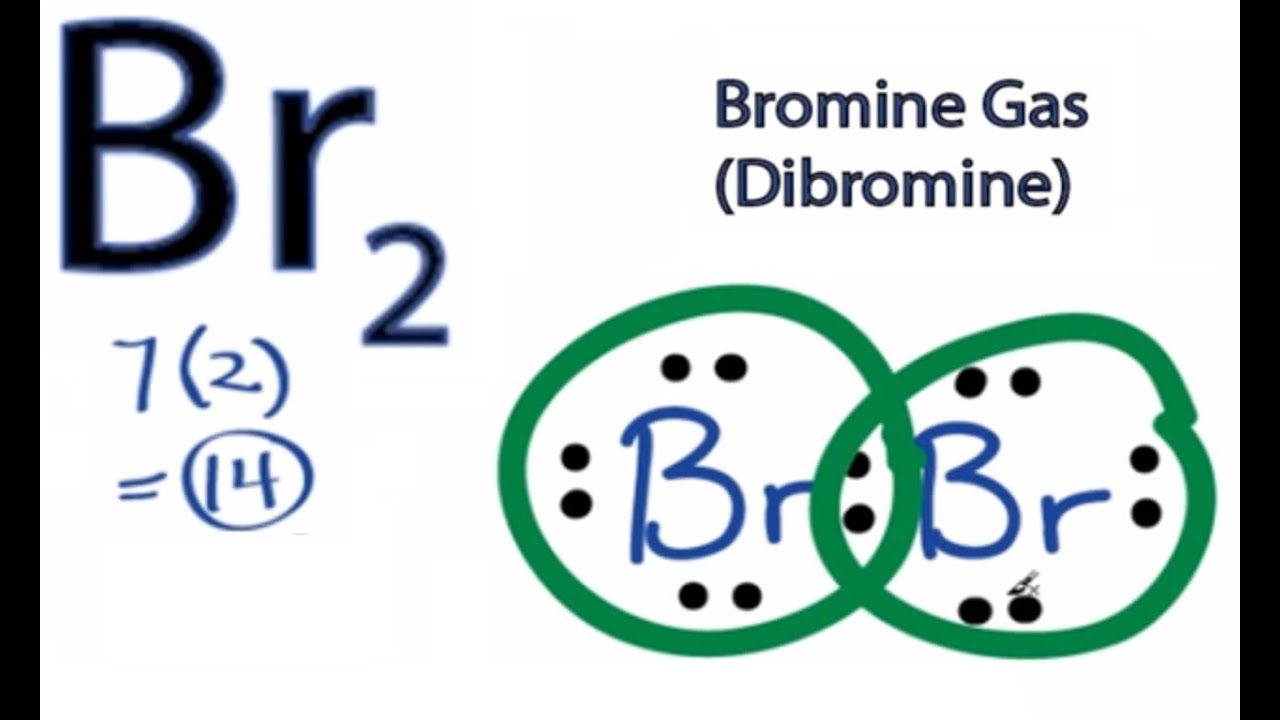
Lewis dot structures can be drawn to show the valence electrons that surround an atom itself. Lewis structures are a useful way to summarize certain information about bonding and may be thought of as electron bookkeeping. In the periodic table the elements are placed in periods and arranged left to right in the order of filling of electrons in the outer shell. Drawing the lewis structure for br 3 viewing notes.
Common chemical compounds are also provided for many elements. The number of dots equals the number of valence electrons in the atom. This means that the lewis dot structure has two dots above below and to the left and one dot to the right.