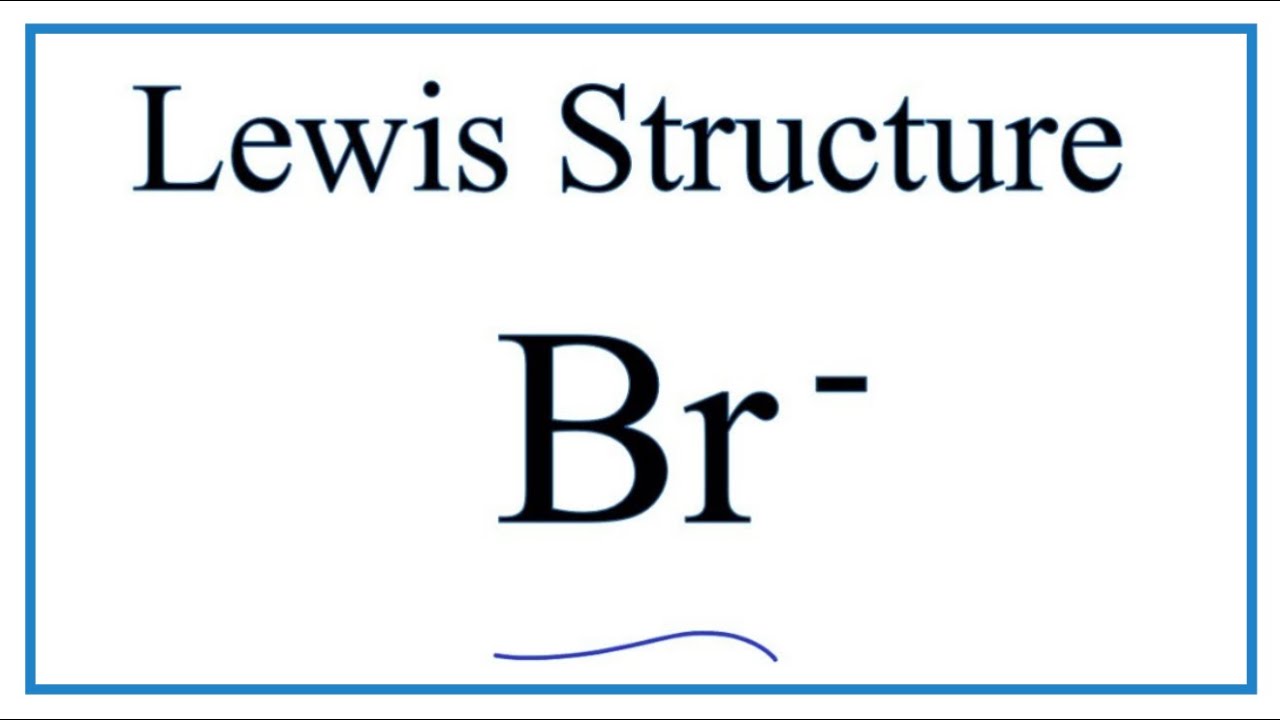
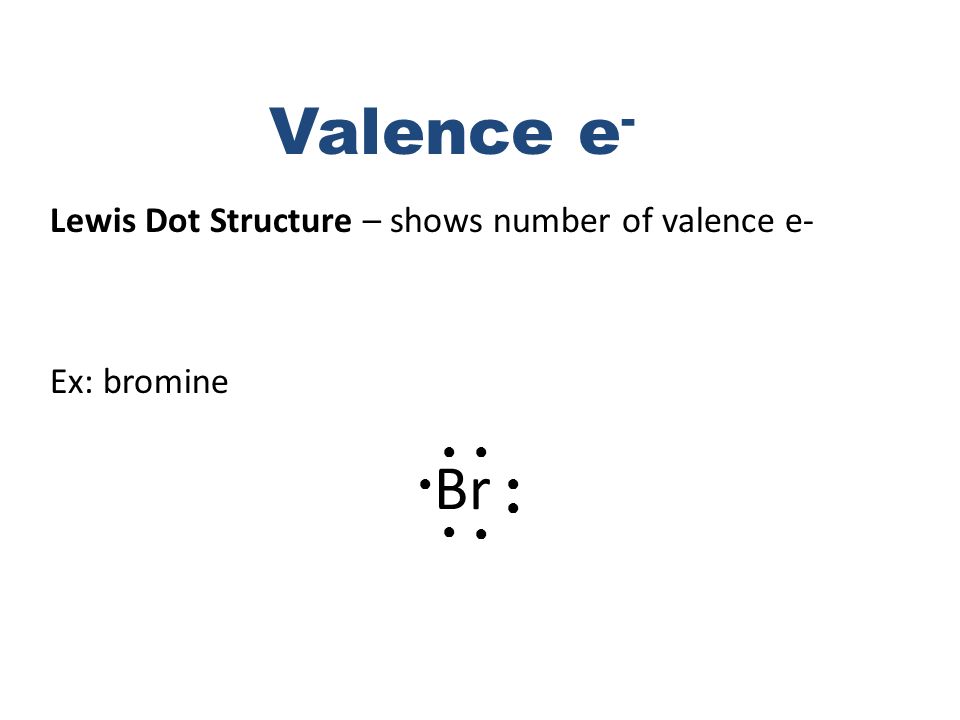
Br Br Lewis Structure
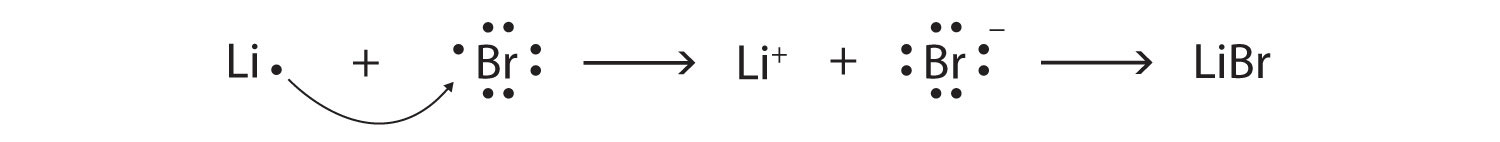
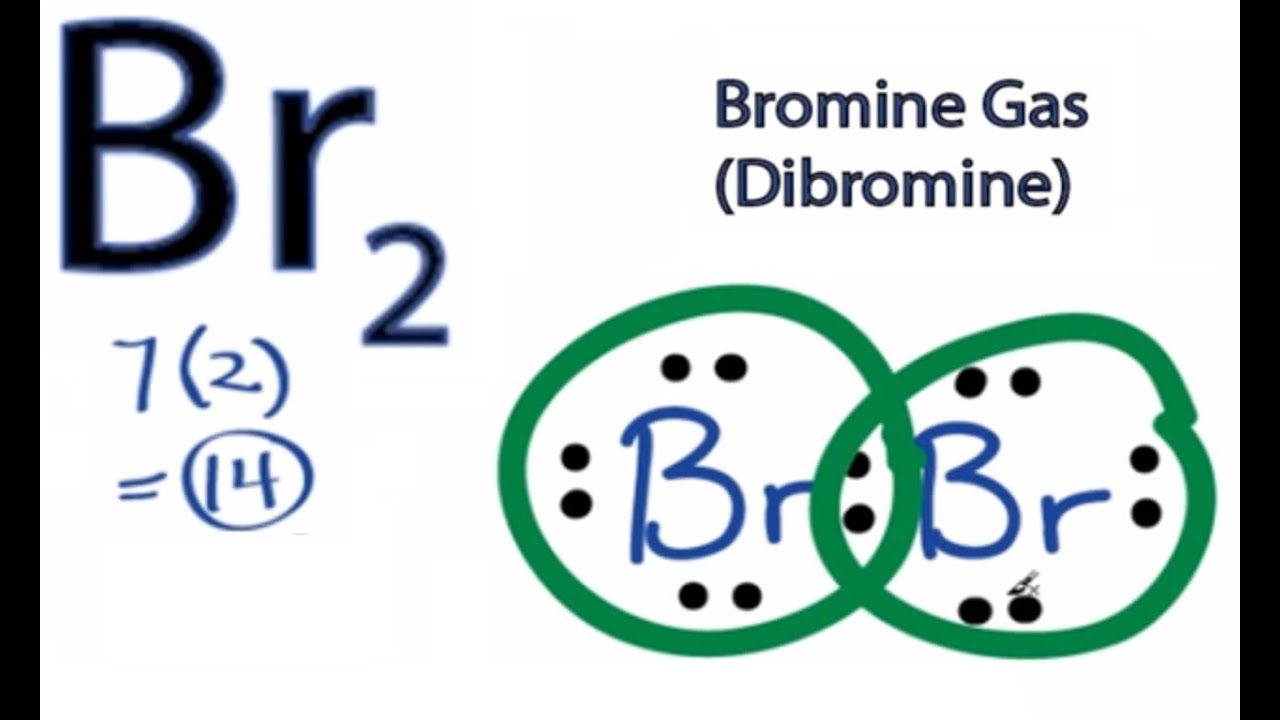
Br 2 is a reddish gas at room temperature.
Br br lewis structure. Br 2 is sometimes called diatomic bromine since it is made up of two bromine atoms. For the br structure use the periodic table to find the total number of valence electro. For the br 2 lewis structure there are a total of 14 valence electrons available. The br 2 lewis structure is similar to f 2 cl 2 and i 2 since f cl and i are all in group 7 and have 7 valence electrons.
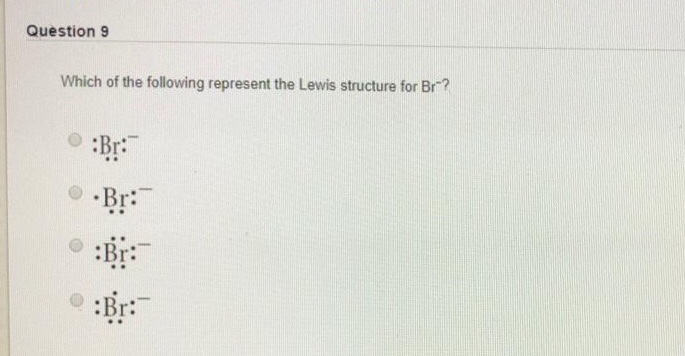
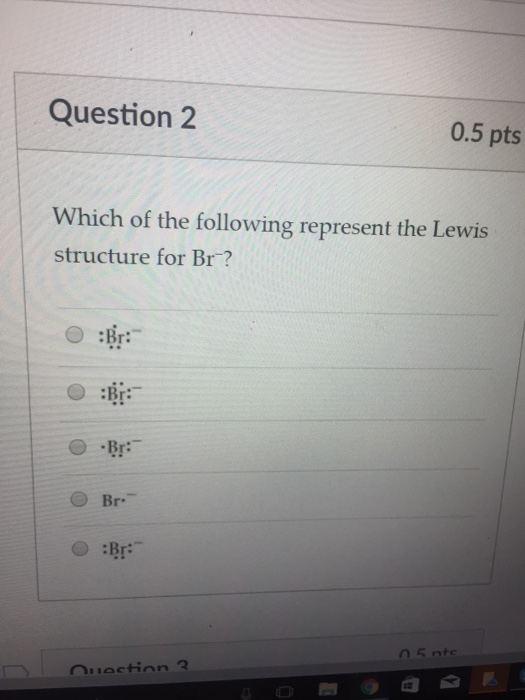
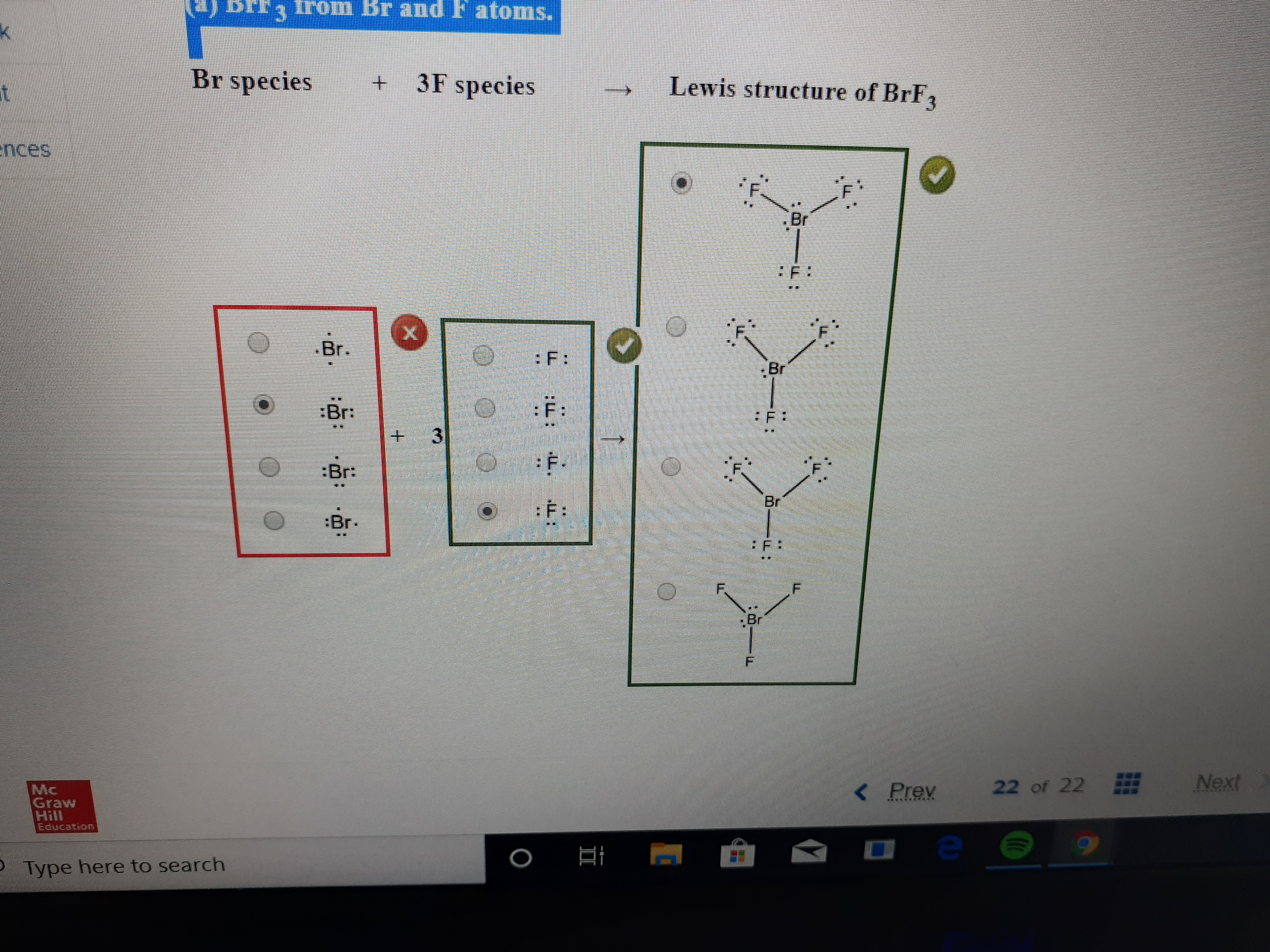
A step by step explanation of how to draw the br lewis dot structure. These 4 pairs of electrons surround the br atom. The br 3 lewis structure has a total of 22 valence electrons. Be sure to put brackets and a negative sign around the dot structure to show that br 3 is an ion with negative one charge.