Br 35 Periodic Table

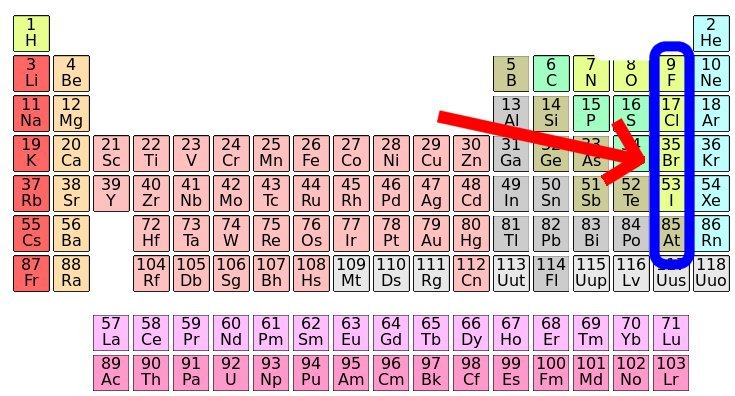
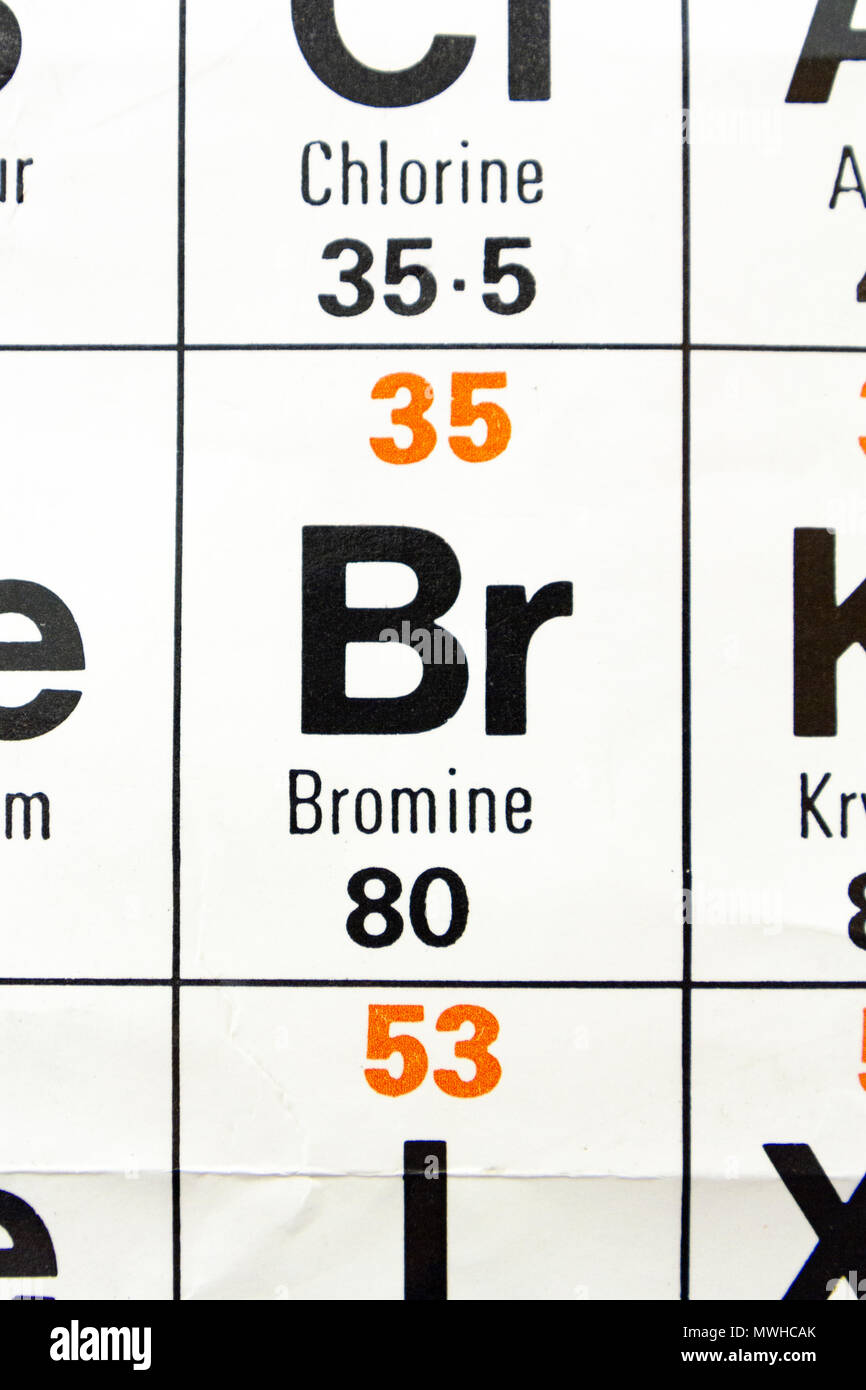
Bromine is a chemical element with atomic number 35 which means there are 35 protons and 35 electrons in the atomic structure.
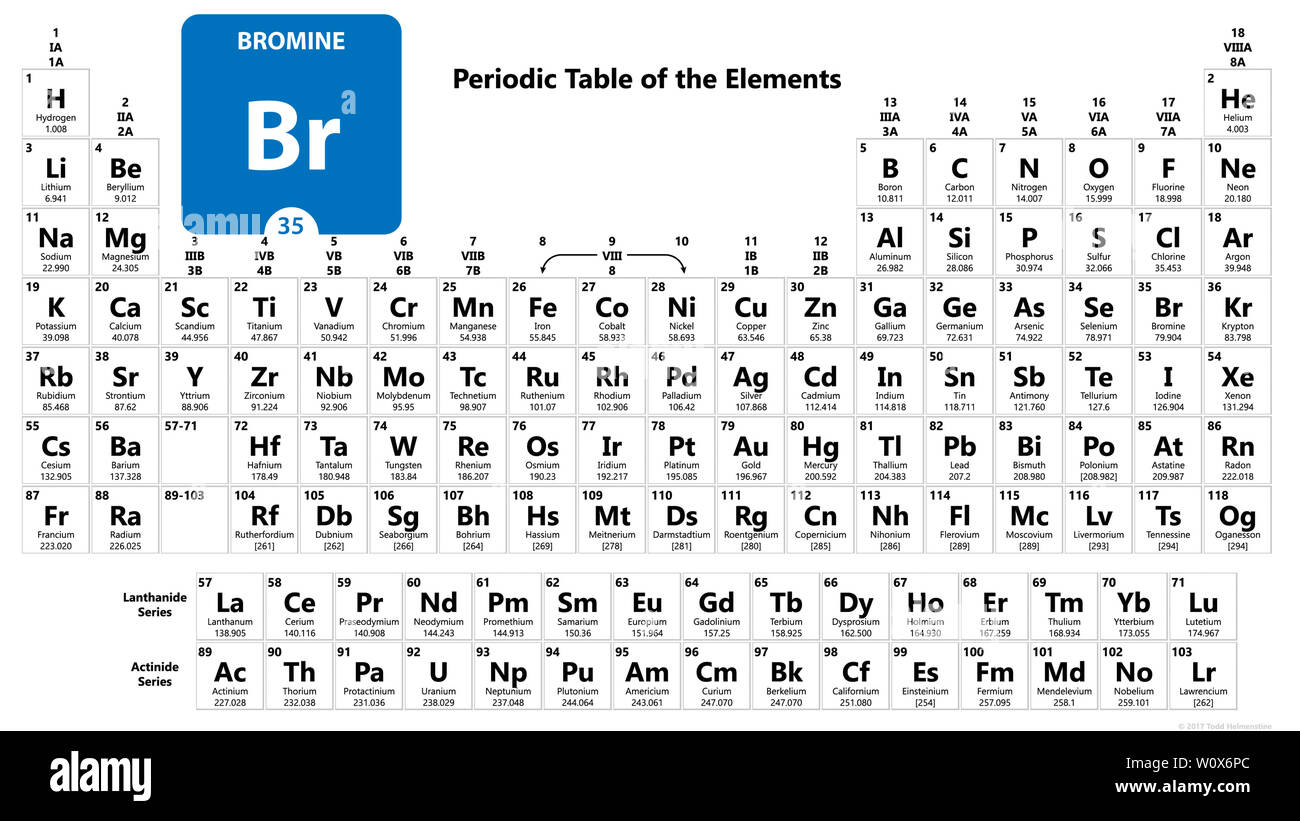
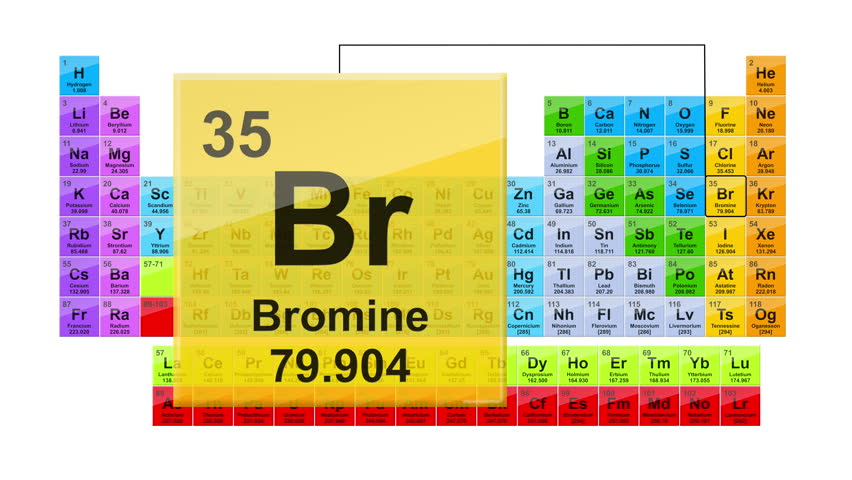
Br 35 periodic table. Bromine is the third lightest halogen and is a fuming red brown liquid at room temperature that evaporates readily to form a similarly coloured gas. The elemental classification of bromine is diatomic nonmetals and it belongs to group 17 and period 4 in the periodic table of elements. The chemical symbol for bromine is br. The melting point of bromine is 265 8 kelvin 7 2.
The relative atomic mass of bromine is 79 904 u. Get the facts about element bromine br 35 from the periodic table. The density of bromine is 3119 kg m 3. Bromine is a heavy mobile brownish red nonmetallic liquid element.
Bromine is extracted by electrolysis from natural bromine rich brine deposits in the usa israel and china. It is volatilizing readily or evaporates easily at room temperature to a red vapor with a strong disagreeable suffocating odor resembling that of chlorine. The chemical symbol for bromine is br. Bromine is a 35.
It has 35 protons and 35 electrons in the atomic structure. Chemical element in the periodic table of elements. It was the first element to be extracted from seawater but this is now only economically viable at the dead sea israel which is particularly rich in bromide up to 0 5. Find physical data electron configuration chemical properties aggregation states isotope data including decay trees as well as some historic information.